Under conditions where no lead loss or gain from the outside environment has occurred, the age of the zircon rock can be calculated by the equations for exponential decay like so:
where in this situation:
 is the number of Uranium atoms measured now.
is the number of Uranium atoms measured now. is the number of Uranium atoms originally - equal to the sum of Lead and Uranium atoms measured now.
is the number of Uranium atoms originally - equal to the sum of Lead and Uranium atoms measured now.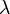 is the decay rate of Uranium.
is the decay rate of Uranium.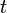 is the age of the zircon rock, which one wants to determine.
is the age of the zircon rock, which one wants to determine.
The above equation is thus equal to:
Which can be rearranged:
and simplified:
The more common form of the equations finally are:
-
 (1)
(1)
and
-
 (2)
(2)
According to Wikipedia, https://en.wikipedia.org/wiki/Uranium-lead_dating, this is the formula for Uranium-Lead dating. However, there is another way to put it that is simpler and works as well. The formula is (1/(2^x))=(Percentage of Uranium in the Zircon). Once you solve for X, multiply the X value by the half life and your answer is the age of the Earth approximately. This method is less exact, but it is much simpler and is very close to the age of the Earth.
However, there are four main problems with this method. First is the fact that we may not have found the oldest Zircon that still exists. Second is the fact that older Zircon may have been destroyed by any number of possible natural or man made forces. Third, if the Zircon that is being examined has been exposed to radioactivity throughout its existence, then the method is ruined because Uranium was added to the Zircon. Lastly, there may be more effective ways of measuring the age of the Earth that we have not discovered yet. These are the main reason why we do not know the exact age of our planet, and we never will. But one of you readers may be the one to discover the Earth's exact age.
Follow on twitter at Planet Earth and visit the Facebook page at Let's Give The World A Chance. Don't forget to like, rate, and subscribe, oh wait, this isn't YouTube. Anyway, stay green Earth, see you all next post.
Follow on twitter at Planet Earth and visit the Facebook page at Let's Give The World A Chance. Don't forget to like, rate, and subscribe, oh wait, this isn't YouTube. Anyway, stay green Earth, see you all next post.



No comments:
Post a Comment